How to set up a Medical company in Dubai

Setting up a Medical Company in the United Arab Emirates (UAE) involves navigating through specific regulations and procedures related to the healthcare industry. Here is a general guide to help you understand the key steps involved:
Market Research and Feasibility Study:
- Conduct a thorough market research and feasibility study to understand the demand for pharmaceutical products in the UAE. Identify potential competitors, target market segments, and regulatory requirements.
Licensing Requirements:
Requirements for Individual Ownership:
- New license application form (will be shared upon confirmation)
- Colored scanned double-page passport (front and back)
- Digital passport photo
- Emirates ID if there is any
- If the shareholder has a visa under a company, then an NOC is required
Requirements for Corporate Ownership:
- Official Certificate: This is an official certificate issued by the relevant authority in the country where the parent company is registered. It should include details such as the name and date of establishment of the company, legal status, capital, business activities, and names of the company’s owners.
- Memorandum of Association (MOA) / Articles of Association (AOA): These documents outline the company’s internal regulations, structure, and governing principles.
- Passport Copies: Copies of the passports of every shareholder and general manager associated with the parent company.
- Board Resolution: A board resolution is needed, and it should specify the following details:
- The decision to establish a representative company in Dubai.
- The names of the appointed manager(s) for the representative company in Dubai, United Arab Emirates (UAE).
- The power of attorney granted to the appointed manager(s), including the specific capacities assigned to each manager, such as:
- c-1. Authorized signatory for opening and closing the company bank account, conducting bank transactions, and signing cheques.
- c-2. The manager is responsible for overseeing the company’s operations.
- c-3. The manager’s name(s) should appear on the trade license (one or more managers).
- The power of attorney given to the authorized person to act on behalf of the parent company, sign all related documents for setting up the representative company,
- select suitable activities, and sign any agreements.
- Minutes of Meeting
IMPORTANT NOTE:
Please be aware that if the parent company is incorporated outside the UAE, the following documents require notarization in the country of origin and attestation by the UAE embassy. After that, they must be attested again in the UAE. The documents must be submitted in English or Arabic. If the original documents are in another language, certified translations will be required for submission. The documents that need notarization and attestation are:
- Board Resolution
- Official Certificate
- Memorandum of Association (MOA) / Articles of Association (AOA)
- Power of Attorney
- Minutes of Meeting
- Additionally, any other relevant documents pertaining to the company should be included in the submission.
Legal Structure and Licensing:
- Choose the legal structure for your pharmaceutical company (e.g., Free Zone Company, DED Mainland). The legal structure will impact licensing requirements.
- Obtain the necessary licenses from the relevant authorities, including Dubai Health Authority (DHA).
Local Partner or Agent:
- For a medical facility incorporation, a local partner or agent is not required we still have to monitor for any changes in the regulations. This partner can be an individual or a company, and their involvement is often mandatory for certain business activities.
Incorporation and Activity:
- Incorporation a new license requires activity and with this, the services to provide will be determined, such as the sample below:
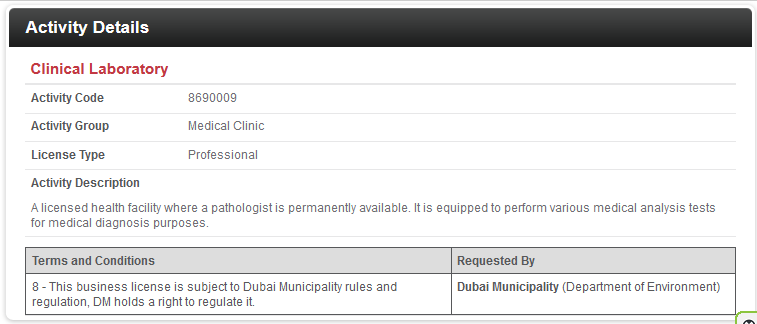
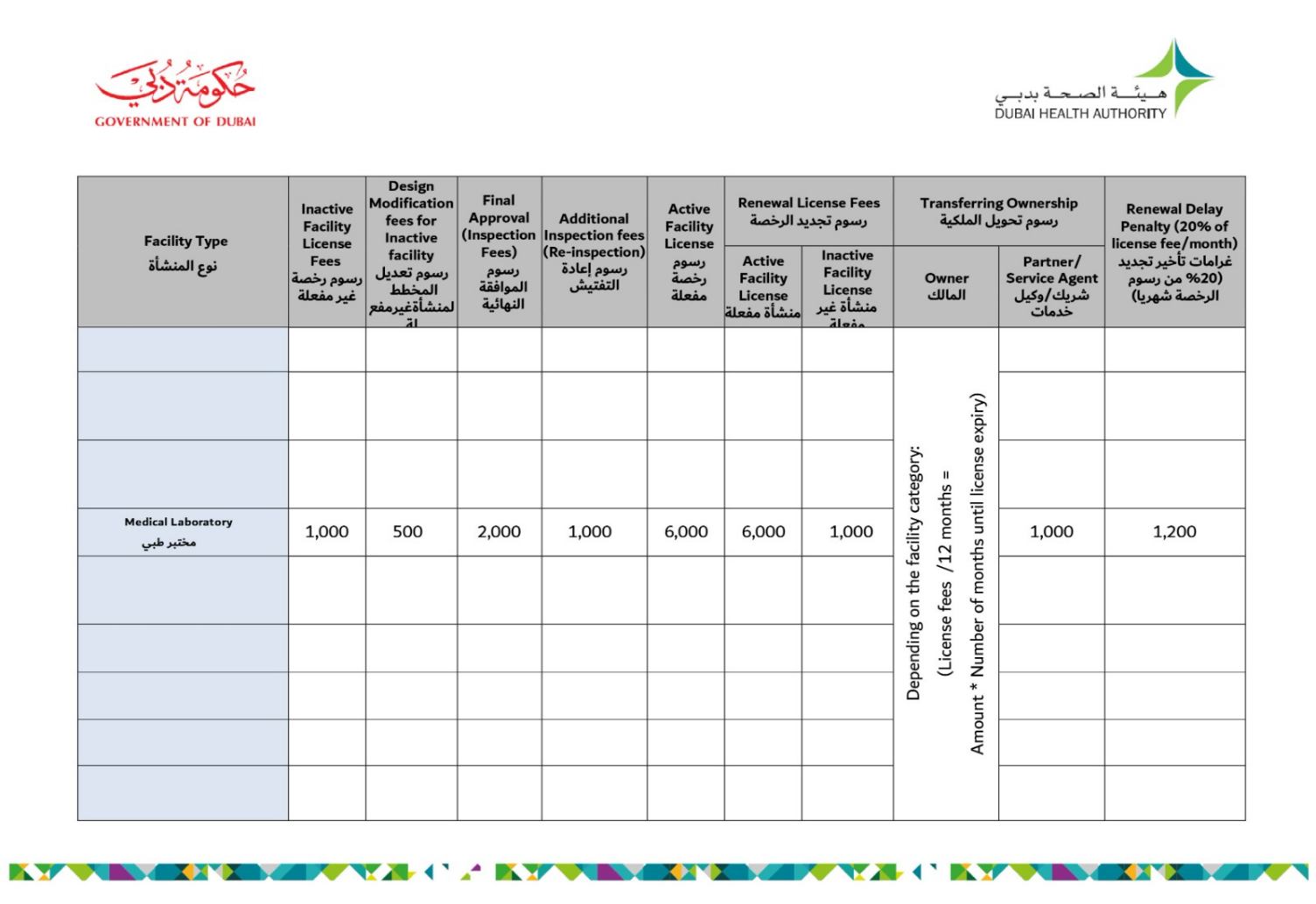
Dubai Health Authority Approval Requirements and Fees:
(This stage is after generating the initial approval.)
Average Processing Time: 10 working days
Required Documents
- Owner/ Partners valid passport copies (and Emirates ID if applicable)
- Engineering Layout from an engineering/design company
- Prepare facility proposal to be filled in the application form (to be shared once confirm)
- Trade license (if available)
- Licensed Laboratory Personnel
Product Registration:
- Register any medical products with the DHA or UAE Ministry of Health and Prevention and Dubai Municipality. This process involves submitting documentation related to the safety, efficacy, and quality of the products.
Distribution and Logistics:
- Establish a reliable distribution network for your pharmaceutical products. Ensure compliance with regulations related to storage, transportation, and distribution.
- Work with licensed distributors and pharmacies to sell and distribute your products.
Employment and Staffing:
- Recruit qualified personnel, including pharmacists, quality control experts, and administrative staff.
- Comply with UAE labor laws regarding employment contracts, working hours, and other relevant regulations.
Approximate Licensing Fees:
| BUSINESS SET-UP (MEDICAL LABORATORY) | |||
| Srl. | Description | Approx. Government Fees (in AED) | PRO Service Charge & Sponsorship Fees
(in AED) |
| 1 | DED Trade license fees | AED 17,000 | |
| 2 | Market Fees | 5% of the Ejari | |
| 3 | Sponsorship Fees | To be confirm | |
| 4 | Service Charge Fees of Business Set-up | To be confirm | |
Note: Ejari, is mandatory for a medical facility.




