How to Set Up a Medical Company in the UAE: A Complete Guide

The United Arab Emirates (UAE) is one of the fastest-growing healthcare markets globally, driven by a rapidly expanding population, world-class infrastructure, and substantial government investment in the medical sector. For entrepreneurs, investors, and healthcare professionals, the opportunity to start a medical company in the UAE is not only lucrative—it is strategically sound.
At Dahhan Business Services, we specialize in guiding clients through the business setup for a medical company across the UAE’s dynamic regulatory landscape. This comprehensive guide outlines everything you need to know about setting up a medical clinic business, including licensing, approvals, location selection, and ongoing compliance.
Why Set Up a Medical Company in the UAE?
Setting up a medical company in the UAE provides access to one of the world’s most modern, well-funded, and patient-focused healthcare ecosystems. Here’s why entrepreneurs are investing heavily in this sector:
- Growing healthcare demand from both locals and expatriates
- Mandatory health insurance policies create stable patient inflows
- Government incentives for foreign healthcare providers
- Technological advancements in telemedicine and digital health
- Strategic location for medical tourism in the GCC and beyond
The UAE Vision 2031 outlines ambitious plans for improving life expectancy and health outcomes, offering even greater growth potential for those entering the healthcare space now.
Types of Medical Companies You Can Set Up
The UAE allows you to establish a variety of healthcare-related entities depending on your business model and expertise:
- Polyclinic or General Practice Medical Centers
- Specialist Clinics (e.g., Dermatology, Cardiology)
- Day Surgery Centers
- Dental Clinics
- Physiotherapy and Rehab Clinics
- Diagnostic Labs and Imaging Centers
- Telemedicine and Digital Health Platforms
- Pharmaceutical and Medical Equipment Trading Companies
Whether you plan to open a small clinic or a multi-specialty hospital, the UAE provides the necessary regulatory and infrastructure support for your medical center business setup.
Steps to Business Setup for a Medical Company in the UAE
Starting a medical company involves multiple legal, operational, and compliance-related steps. With expert help from Dahhan Business Services, you can navigate this process efficiently.
Step 1: Choose the Business Jurisdiction
The first decision is whether to set up in the mainland, free zone, or offshore. For a medical company, mainland jurisdictions like the Dubai Health Authority (DHA) and the Department of Health Abu Dhabi (DOH) are commonly chosen.
Free zones, such as Dubai Healthcare City (DHCC) and Sharjah Healthcare City (SHCC), also offer special incentives for medical professionals and investors.
Step 2: Determine the Legal Structure
Depending on your goals and capital, choose from the following legal structures:
- Sole Proprietorship (for licensed healthcare professionals)
- Limited Liability Company (LLC) (for partnerships with up to 50 shareholders)
- Private Healthcare Company
- Branch of a Foreign Medical Company
Each legal form has specific requirements regarding ownership, liability, and local sponsorship.
Step 3: Select a Suitable Business Activity and Trade Name
The Department of Economic Development (DED) or the relevant free zone authority will classify your medical clinic business setup under a specific activity code. Select the correct medical service type and ensure your trade name complies with the UAE’s naming regulations.
Dahhan Business Services assists with:
- Verifying the availability of your trade name
- Ensuring name alignment with your medical practice
- Reserving your trade name quickly
Step 4: Obtain Initial Approvals
Before moving forward, you need to secure pre-approvals from:
- Ministry of Health and Prevention (MOHAP)
- Dubai Health Authority (DHA)
- Department of Health (DOH) (for Abu Dhabi-based clinics)
- Health Authority of Sharjah (for clinics in Sharjah)
Initial approval requires submitting:
- Passport copies of shareholders
- Professional qualification certificates
- Proposed facility layout
- Business plan
Step 5: Secure a Commercial Space and Facility Design Approval
You must lease or purchase a commercial space that meets the strict standards of a healthcare facility. The location should meet:
- Space and hygiene regulations
- Emergency access and parking
- Zoning and noise isolation standards
- Infrastructure for medical equipment
DHA or the relevant health authority must review and approve your interior layout and design before issuing a license.
Step 6: Obtain the Medical License and Commercial License
After passing inspections and receiving all approvals, your business setup medical clinic is finalized with two licenses:
- Medical License – issued by DHA, DOH, or MOHAP, depending on location
- Commercial License – issued by DED or the relevant free zone authority
These licenses enable you to provide healthcare services and operate your facility in a legally compliant manner.
Required Documents for Medical Clinic Business Setup
The documentation required may vary by emirate and authority, but generally includes:
- Passport copies of all shareholders
- Visa page and Emirates ID (for UAE residents)
- Degree certificates and professional qualifications
- Facility lease contract (Ejari for Dubai)
- No Objection Certificate (NOC) from current sponsor (if applicable)
- Medical license application forms
- Business plan
- Floor plan and facility design approvals
- Payment of relevant license and inspection fees
Dahhan Business Services streamlines this entire process for you—preparing, submitting, and following up on all required documentation.
Cost of Setting Up a Medical Company in the UAE
The cost to set up a medical company depends on the emirate, type of facility, and services offered. On average, the breakdown includes:
Expense Approximate Cost (AED)
Trade Name Registration 1,000 – 2,000
Initial Approvals 2,000 – 5,000
Commercial License 10,000 – 15,000
Medical License 8,000 – 15,000
Facility Fit-Out and Equipment 100,000 – 500,000+
DHA/DOH Registration Fees 5,000 – 10,000
Costs may vary significantly based on location, clinic size, and licensing type. Contact our team for a detailed cost estimate customized to your business model.
Benefits of Medical Center Business Setup in the UAE
- 100% foreign ownership in many free zones
- Ease of capital repatriation and tax-free profits
- Growing demand for healthcare services
- Diverse patient base and international clientele
- Modern infrastructure and cutting-edge medical tech
- Support from the UAE government and regulatory bodies
- Opportunities in telemedicine and digital health
With the proper guidance, your business setup for a medical company can be fast-tracked to success.
How Dahhan Business Services Supports Your Medical Business Setup
At Dahhan Business Services, we provide a customized approach to establishing your medical company in the UAE. Our services cover every step of the journey:
✅ Business Structuring and Licensing
We help you choose the proper jurisdiction, legal form, and business activity that suits your goals.
✅ Approvals from Health Authorities
We liaise directly with DHA, DOH, MOHAP, and free zone authorities to secure all necessary approvals.
✅ Facility Layout and Interior Design Compliance
Our team connects you with health-approved contractors and ensures your clinic complies with design standards.
✅ Commercial Space Search
We assist in finding an approved clinic location that meets licensing and operational requirements.
✅ Medical Staff Licensing
Support with doctor and nurse licensing, dataflow verification, and examination scheduling for medical professionals.
✅ PRO and Visa Services
We handle your clinic’s investor visas, employee visas, and Emirates ID applications quickly and professionally.
Contact Us!
Ready to open your medical company in the UAE?
Dahhan Business Services has helped numerous clients launch clinics, laboratories, and healthcare businesses in Dubai, Abu Dhabi, Sharjah, and beyond. From licensing to facility design, we handle everything so you can focus on healing lives and growing your business.
📞 Contact us today for a free consultation with our business setup experts.
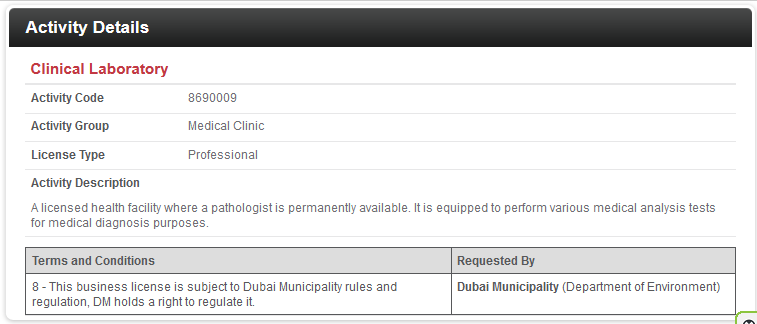
Incorporation and Activity:
Incorporation a new license requires activity, and with this, the services to provide will be determined, such as the sample below:
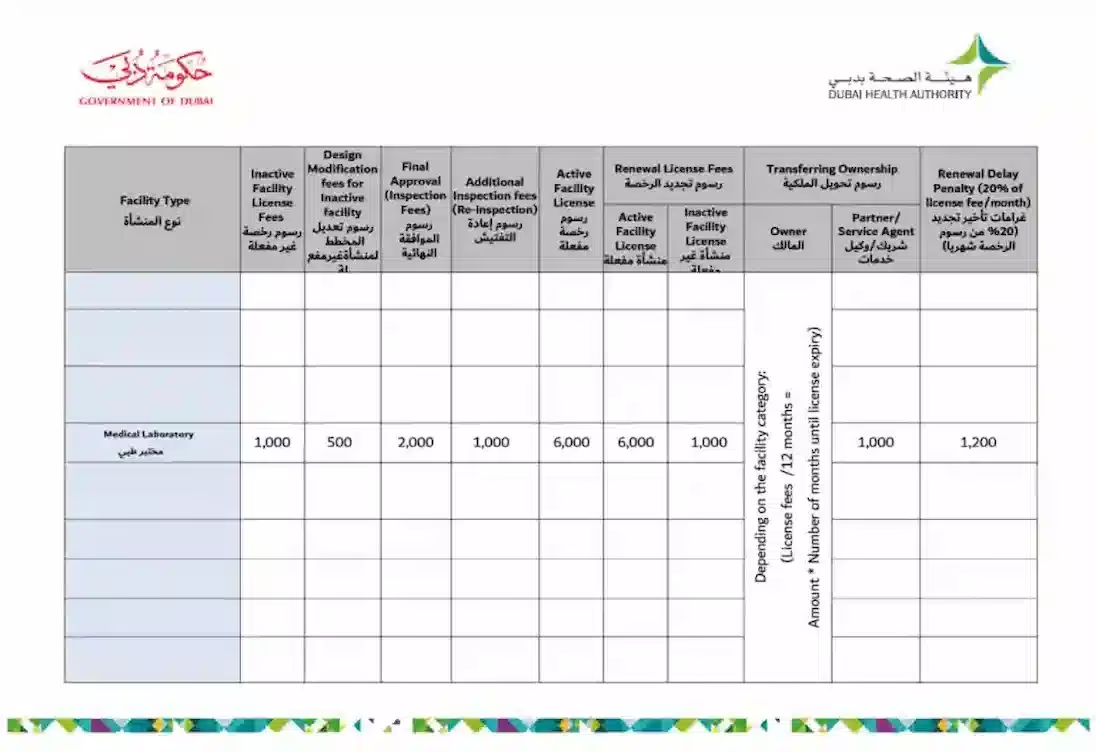
Dubai Health Authority Approval Requirements and Fees:
(This stage is after generating the initial approval.)
Average Processing Time: 10 working days
Required Documents
- Owner/ Partners valid passport copies (and Emirates ID if applicable)
- Engineering Layout from an engineering/design company
- Prepare facility proposal to be filled in the application form (to be shared once confirm)
- Trade license (if available)
- Licensed Laboratory Personnel
Product Registration:
- Register any medical products with the DHA or UAE Ministry of Health and Prevention and Dubai Municipality. This process involves submitting documentation related to the safety, efficacy, and quality of the products.
Distribution and Logistics:
- Establish a reliable distribution network for your pharmaceutical products. Ensure compliance with regulations related to storage, transportation, and distribution.
- Work with licensed distributors and pharmacies to sell and distribute your products.
Employment and Staffing:
- Recruit qualified personnel, including pharmacists, quality control experts, and administrative staff.
- Comply with UAE labor laws regarding employment contracts, working hours, and other relevant regulations.
Approximate Licensing Fees:
| BUSINESS SET-UP (MEDICAL LABORATORY) | |||
|---|---|---|---|
| Srl. | Description | Approx. Government Fees (in AED) | PRO Service Charge & Sponsorship Fees (in AED) |
| 1 | DED Trade license fees | AED 17,000 | |
| 2 | Market Fees | 5% of the Ejari | |
| 3 | Sponsorship Fees | To be confirm | |
| 4 | Service Charge Fees of Business Set-up | To be confirm | |
Note: Ejari, is mandatory for a medical facility.
Frequently Asked Questions (FAQ)
What are the legal requirements to open a medical company in the UAE?
You need a valid medical license from the local health authority (DHA, DOH, or MOHAP) and a commercial license from the DED or free zone. A qualified medical director must be appointed.
Can foreigners open a medical clinic in Dubai?
Yes. Foreign investors can open a medical clinic in Dubai with 100% ownership in approved zones or through local partnerships in mainland areas.
How long does it take to set up a medical clinic business?
The timeline ranges from 4 to 8 weeks, depending on the approvals required, facility readiness, and the completeness of documentation.
Is telemedicine legal in the UAE?
Yes. Telemedicine is legal and regulated in the UAE. You can obtain a specialized license for operating a digital health platform or remote consultation service.
What is the minimum investment to start a clinic in Dubai?
A small medical clinic may require AED 150,000 to AED 300,000, while specialized or multi-disciplinary centers can go up to AED 1 million or more.
Final Thoughts
The UAE is poised to become a global healthcare hub, and now is the perfect time to invest in a medical company. With the right licensing, infrastructure, and business setup support, you can tap into one of the region’s most promising and sustainable industries.
Whether you’re a doctor opening a private practice, an investor launching a healthcare facility, or a multinational expanding into the GCC, Dahhan Business Services is your trusted partner for setting up a medical center in the UAE.
Let us help you build a compliant, profitable, and respected medical clinic that meets the healthcare needs of a thriving population.
Interested in starting your medical company in Dubai, Abu Dhabi, or Sharjah?
👉 Get in touch with Dahhan Business Services today to begin your journey.





